TOC ANALYSIS OF LIQUIDS BY COMBUSTION AND COULOMETRIC DETECTION
CM130 TC AND TOC ANALYSIS OF LIQUIDS BY COMBUSTION AND COULOMETRIC DETECTION
TC And TOC Analysis Of Liquids By Combustion And Coulometric Detection
Applications include: Water and wastewater, brines, process fluids, corrosive agents and acids.
The CM130 Total Carbon Analyzer is a complete analytical system capable of measuring total carbon and total organic carbon in aqueous samples. Combining a high-temperature combustion furnace with a highly sensitive CO2 detector, the CM130 is capable of analyzing samples containing carbon concentrations from ppm levels to 10,000 µg C (absolute) without user calibration. UIC’s analyzers are rugged, accurate and adaptable to most TC/TOC applications. They are used extensively in industrial, research and educational laboratories worldwide. The CM130 system includes the following components pictured above:
CM5017 CO2 Coulometer
- No user calibration
- Wide, linear dynamic range
- Readability to 0.01 µg Carbon
- User selectable display units
- 10″ LCD Touch Screen
- SD Card data storage
- LIMS Compatible
CM5300 Horizontal Furnace with CM5321 Furnace Kit
- Programmable up to 1100OC
- Pre-combustion scrubbers for removal of interferences from oxygen carrier gas
- Post-combustion scrubbers for removal of interfering gases formed during sample combustion
- Sample introduction using platinum or porcelain boats with ladle
Instrument Capabilities
A major advantage of the CM130 TC/TOC Analyzer is the use of coulometric detection. Employing the principles of Faraday’s Law, the CM5017 CO2 Coulometer automatically measures the absolute mass amount of carbon dioxide resulting from sample combustion. No user-calibration is required and linear detection is available from less than 1 µg carbon to over 10,000 µg carbon. Using this 100% efficient coulometric process, relative standard deviations of 0.2% or better are common for standard material. For smaller concentrations, an absolute deviation of approximately 1 µg C is typical.
Users with samples containing particulates, solids or solid/liquid slurries should consider the CM120 or CM135 systems offered by UIC. Oxidation times vary with sample type and temperature although 5 to 7 minute analyses are typical.
Total Carbon
A variable volume, constant rate syringe is used to introduce the sample into the high temperature oxygen atmosphere (typically 950OC) within the sample combustion zone. In that environment, all carbon within the sample is rapidly oxidized to CO2. Interfering reaction products (including sulphur oxides, halides, water and nitrous oxides) are removed by the post-combustion scrubbers. The resulting carbon dioxide is then swept into the CM5017 CO2 Coulometer where it is automatically measured using absolute coulometric titration.
Total Organic Carbon
Prior to injection into the furnace, the sample is acidified and purged of CO2 and carbonate carbons. This “pre-treated” sample is then reacted as described above.
Data Handling
Names, weights and sizes of up to 50 samples can be entered, to be used by the CM5017 in calculating the final result. Analytical progress is displayed on the 10” LCD touch screen in user-selectable units. Detailed analysis information is automatically saved to an on-board SD card after each sample. Data can also be transmitted through the standard serial and Ethernet ports to be captured on a personal computer or LIMS. In addition, a detailed report can be printed to the optional small format printer while each sample is running.
Applications include: Water and wastewater, brines, process fluids, corrosive agents and acids.
The CM130 Total Carbon Analyzer is a complete analytical system capable of measuring total carbon and total organic carbon in aqueous samples. Combining a high-temperature combustion furnace with a highly sensitive CO2 detector, the CM130 is capable of analyzing samples containing carbon concentrations from ppm levels to 10,000 µg C (absolute) without user calibration. UIC’s analyzers are rugged, accurate and adaptable to most TC/TOC applications. They are used extensively in industrial, research and educational laboratories worldwide. The CM130 system includes the following components pictured above:
CM5017 CO2 Coulometer
|
CM5300 Horizontal Furnace with CM5321 Furnace Kit
|
Instrument Capabilities
A major advantage of the CM130 TC/TOC Analyzer is the use of coulometric detection. Employing the principles of Faraday’s Law, the CM5017 CO2 Coulometer automatically measures the absolute mass amount of carbon dioxide resulting from sample combustion. No user-calibration is required and linear detection is available from less than 1 µg carbon to over 10,000 µg carbon. Using this 100% efficient coulometric process, relative standard deviations of 0.2% or better are common for standard material. For smaller concentrations, an absolute deviation of approximately 1 µg C is typical.
Users with samples containing particulates, solids or solid/liquid slurries should consider the CM120 or CM135 systems offered by UIC. Oxidation times vary with sample type and temperature although 5 to 7 minute analyses are typical.
Total Carbon
A variable volume, constant rate syringe is used to introduce the sample into the high temperature oxygen atmosphere (typically 950OC) within the sample combustion zone. In that environment, all carbon within the sample is rapidly oxidized to CO2. Interfering reaction products (including sulphur oxides, halides, water and nitrous oxides) are removed by the post-combustion scrubbers. The resulting carbon dioxide is then swept into the CM5017 CO2 Coulometer where it is automatically measured using absolute coulometric titration.
Total Organic Carbon
Prior to injection into the furnace, the sample is acidified and purged of CO2 and carbonate carbons. This “pre-treated” sample is then reacted as described above.
Data Handling
Names, weights and sizes of up to 50 samples can be entered, to be used by the CM5017 in calculating the final result. Analytical progress is displayed on the 10” LCD touch screen in user-selectable units. Detailed analysis information is automatically saved to an on-board SD card after each sample. Data can also be transmitted through the standard serial and Ethernet ports to be captured on a personal computer or LIMS. In addition, a detailed report can be printed to the optional small format printer while each sample is running.
COMPARISON BETWEEN COULOMETRIC DETECTION AND NDIR DETECTION METHOD
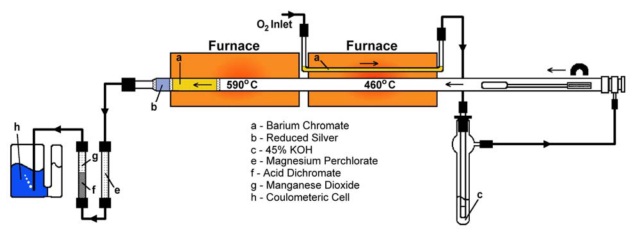
Whether using a coulometric or NDIR detection system when analyzing samples for carbon content, both detectors actually detect carbon dioxide (CO2). The carbon analysis instrument will utilize either combustion, acidification, ultraviolet light or a persulfate to convert carbon into CO2. A CO2-free carrier gas is used to transport the sample gas to the detector. This is generally where the similarities end.
The coulometric detector is known as an “absolute” detection method. This means that there is a one-to-one relationship between the number of carbon atoms in the sample and the detector response. This provides a linear detector response and allows the coulometric detection method to be considered “calibration free”. That is, the detector does not need to be calibrated over its entire analytical range in order to provide accurate results.
The coulometric detector is simply conducting an acid-base titration and can be likened to using a burette to add a base to an acidic solution to determine the amount of acid in the solution. In the coulometric cell, CO2 enters the solution and reacts with an amine to form an acid. The solution also contains an indicator that is blue in color at the endpoint. As acid forms, the blue indicator begins to fade. A light path through the cell monitors the percent transmittance (%T) through the cell and, as the blue color fades, acts as a switch to turn on the cell current.
The cell current is used to generate a base by electrolyzing water to H+ and OH-. The OH- ion reacts with the acidic solution formed by the CO2 until the solution is neutralized back to the endpoint. The amount of current used to generate the base is directly proportional to the amount of CO2 that has entered the cell. We simply integrate the amount of current used into a form that is selected and understood by the user.
NDIR detectors also detect CO2, but in a much different manner. An NDIR detector is composed of a tubular shaped cell with a carrier gas inlet on one end and an outlet on the other end. Also, at one end of the cell is an infrared emitter and at the other end is an infrared detector. The wavelength of the infrared source is chosen so that the infrared light is absorbed by CO2.
As CO2 passes through the cell it absorbs the infrared energy and causes a change in the response of the infrared detector. The NDIR detector must be calibrated with known standards in order to relate the detector response to a particular CO2 concentration. The detector response is not necessarily linear with the concentration and several standards must be used in order to determine the “calibration curve”. We refer to this as a “relative” relationship as opposed to the “absolute” relationship of the coulometric detector.
Whether using a coulometric or NDIR detection system when analyzing samples for carbon content, both detectors actually detect carbon dioxide (CO2). The carbon analysis instrument will utilize either combustion, acidification, ultraviolet light or a persulfate to convert carbon into CO2. A CO2-free carrier gas is used to transport the sample gas to the detector. This is generally where the similarities end.
The coulometric detector is known as an “absolute” detection method. This means that there is a one-to-one relationship between the number of carbon atoms in the sample and the detector response. This provides a linear detector response and allows the coulometric detection method to be considered “calibration free”. That is, the detector does not need to be calibrated over its entire analytical range in order to provide accurate results.
The coulometric detector is simply conducting an acid-base titration and can be likened to using a burette to add a base to an acidic solution to determine the amount of acid in the solution. In the coulometric cell, CO2 enters the solution and reacts with an amine to form an acid. The solution also contains an indicator that is blue in color at the endpoint. As acid forms, the blue indicator begins to fade. A light path through the cell monitors the percent transmittance (%T) through the cell and, as the blue color fades, acts as a switch to turn on the cell current.
The cell current is used to generate a base by electrolyzing water to H+ and OH-. The OH- ion reacts with the acidic solution formed by the CO2 until the solution is neutralized back to the endpoint. The amount of current used to generate the base is directly proportional to the amount of CO2 that has entered the cell. We simply integrate the amount of current used into a form that is selected and understood by the user.
NDIR detectors also detect CO2, but in a much different manner. An NDIR detector is composed of a tubular shaped cell with a carrier gas inlet on one end and an outlet on the other end. Also, at one end of the cell is an infrared emitter and at the other end is an infrared detector. The wavelength of the infrared source is chosen so that the infrared light is absorbed by CO2.
As CO2 passes through the cell it absorbs the infrared energy and causes a change in the response of the infrared detector. The NDIR detector must be calibrated with known standards in order to relate the detector response to a particular CO2 concentration. The detector response is not necessarily linear with the concentration and several standards must be used in order to determine the “calibration curve”. We refer to this as a “relative” relationship as opposed to the “absolute” relationship of the coulometric detector.
Detection Range
Since a single CO2 molecule may be “counted” several times as it passes through the cell, the NDIR detector can be very effective at conducting low-level carbon analyses. Longer cell lengths allow for lower level analysis, often down to the low part-per-billion (ppb) range.
However, with high carbon levels, the NDIR detector is subject to being overloaded, or “swamped”, with CO2. Manufacturers have compensated for this by using shorter cell lengths, splitting the carrier gas stream so that only a portion goes through the cell, using a much smaller sample size and/or using electronic gain control to make the infrared detector less sensitive. Many manufacturers now claim that they can analyze carbon from the low ppb range up to 100% using a combination of these methods. Shimadzu has a published analytical range of 4 ug/L to 30,000 ug/L (4ppb to 3%). They use an auto-dilution feature to dilute the sample from 2 to 50 times in order to achieve the high end of the detection range on liquid samples. It is unclear what process they use for solid samples.
The published analytical range of the coulometric detector is 10ugC to 100mgC per sample, regardless of sample size. Some sample types, such as water, may be limited in sample size due to the nature of the analysis. Performing a Total Carbon (TC) analysis of water via high temperature combustion necessitates a maximum sample size of 200uL. In this example, if we want to calculate the lower detectable limit (LDL) of the instrument, we need to divide the minimum detector response (10ugC) by the sample size (200uL or 0.2mg). So, 10ugC/0.2mg = 50ppmC. However, if we want to determine the total inorganic carbon (TIC) content of a water sample via acidification, we can use a sample size up to 100ml. Therefore, the LDL of a TIC analysis of water would be 10ugC/100g = 0.1ppmC.
Since a single CO2 molecule may be “counted” several times as it passes through the cell, the NDIR detector can be very effective at conducting low-level carbon analyses. Longer cell lengths allow for lower level analysis, often down to the low part-per-billion (ppb) range.
However, with high carbon levels, the NDIR detector is subject to being overloaded, or “swamped”, with CO2. Manufacturers have compensated for this by using shorter cell lengths, splitting the carrier gas stream so that only a portion goes through the cell, using a much smaller sample size and/or using electronic gain control to make the infrared detector less sensitive. Many manufacturers now claim that they can analyze carbon from the low ppb range up to 100% using a combination of these methods. Shimadzu has a published analytical range of 4 ug/L to 30,000 ug/L (4ppb to 3%). They use an auto-dilution feature to dilute the sample from 2 to 50 times in order to achieve the high end of the detection range on liquid samples. It is unclear what process they use for solid samples.
The published analytical range of the coulometric detector is 10ugC to 100mgC per sample, regardless of sample size. Some sample types, such as water, may be limited in sample size due to the nature of the analysis. Performing a Total Carbon (TC) analysis of water via high temperature combustion necessitates a maximum sample size of 200uL. In this example, if we want to calculate the lower detectable limit (LDL) of the instrument, we need to divide the minimum detector response (10ugC) by the sample size (200uL or 0.2mg). So, 10ugC/0.2mg = 50ppmC. However, if we want to determine the total inorganic carbon (TIC) content of a water sample via acidification, we can use a sample size up to 100ml. Therefore, the LDL of a TIC analysis of water would be 10ugC/100g = 0.1ppmC.
Accuracy and Precision
It is difficult to determine the accuracy of infrared detection systems because of the “relative” nature of the analysis. The user must tell the instrument what the theoretical value of the standard material is during the calibration procedure and the instrument relates the detector response to that theoretical value. Reanalyzing that same standard material as a sample should produce a value very close to the original, resulting in what appears to be very good accuracy. Problems can occur with a change to the standard material, user input error and in the number of calibration points established. Most NDIR-based instrument manufacturers do not publish accuracy specifications because of these variables.
The coulometric detector on the other hand is an “absolute” determination of the amount of carbon (or CO2) present in any standard or sample. The detector does not rely on any user input to determine how much carbon is present in a sample. UIC has established an accuracy specification of +/-1.25% of the theoretical value of the standard material. This is, of course, assuming that the material is pure, dry and accurately measured (weighed). Since the response of the coulometric detector is totally linear, there is no need to establish a calibration curve throughout the analytical range. We only use standards as a means of confirming the performance of the instrument.
Precision is a measure of the repeatability of an analysis. This can be called the Coefficient of Variation or the Relative Standard Deviation. In either case, it is calculated by dividing the standard deviation by the mean. Shimadzu has a published precision of “less than 1.5%”. UIC has a published precision of “not more than 0.4%” for standards weighed on an analytical balance and “not more than 0.2%” for standards weighed on a micro-balance. The procedures adopted by Oceanographers to determine the total dissolved inorganic carbon in sea water have resulted in a “within cruise” accuracy of +/-1.5 umol/kg (approximately 0.075%), a “between cruise” accuracy of +/-4 umol/kg (approximately 0.2%) and a relative standard deviation (RSD) of 0.05% (when using a UIC coulometric detection system).
It is difficult to determine the accuracy of infrared detection systems because of the “relative” nature of the analysis. The user must tell the instrument what the theoretical value of the standard material is during the calibration procedure and the instrument relates the detector response to that theoretical value. Reanalyzing that same standard material as a sample should produce a value very close to the original, resulting in what appears to be very good accuracy. Problems can occur with a change to the standard material, user input error and in the number of calibration points established. Most NDIR-based instrument manufacturers do not publish accuracy specifications because of these variables.
The coulometric detector on the other hand is an “absolute” determination of the amount of carbon (or CO2) present in any standard or sample. The detector does not rely on any user input to determine how much carbon is present in a sample. UIC has established an accuracy specification of +/-1.25% of the theoretical value of the standard material. This is, of course, assuming that the material is pure, dry and accurately measured (weighed). Since the response of the coulometric detector is totally linear, there is no need to establish a calibration curve throughout the analytical range. We only use standards as a means of confirming the performance of the instrument.
Precision is a measure of the repeatability of an analysis. This can be called the Coefficient of Variation or the Relative Standard Deviation. In either case, it is calculated by dividing the standard deviation by the mean. Shimadzu has a published precision of “less than 1.5%”. UIC has a published precision of “not more than 0.4%” for standards weighed on an analytical balance and “not more than 0.2%” for standards weighed on a micro-balance. The procedures adopted by Oceanographers to determine the total dissolved inorganic carbon in sea water have resulted in a “within cruise” accuracy of +/-1.5 umol/kg (approximately 0.075%), a “between cruise” accuracy of +/-4 umol/kg (approximately 0.2%) and a relative standard deviation (RSD) of 0.05% (when using a UIC coulometric detection system).
Interferences
Both the coulometric and NDIR methods are subject to interference from other compounds. For the coulometric method it is SO2 and H2S. Both of these compounds will react with the amine in the solution in the same way as CO2, but they are easily scrubbed out of the carrier gas and rarely present any problem. For the NDIR detector H2O absorbs infrared energy in the same wavelength as CO2. Therefore, it is very important to use very dry carrier gas and to not operate in high humidity conditions. Most NDIR manufacturers use a chemical means (magnesium perchlorate) to scrub moisture out of the carrier gas. With either detector the interferences are well known and easily removed.
Both the coulometric and NDIR methods are subject to interference from other compounds. For the coulometric method it is SO2 and H2S. Both of these compounds will react with the amine in the solution in the same way as CO2, but they are easily scrubbed out of the carrier gas and rarely present any problem. For the NDIR detector H2O absorbs infrared energy in the same wavelength as CO2. Therefore, it is very important to use very dry carrier gas and to not operate in high humidity conditions. Most NDIR manufacturers use a chemical means (magnesium perchlorate) to scrub moisture out of the carrier gas. With either detector the interferences are well known and easily removed.
Analysis Times
Infrared-based systems have an average analysis time of around 3 minutes for the determination of TOC by a combustion method. The coulometric-based systems offered by UIC have an average analysis time of about 7 minutes for the same analysis. There will be variations in analysis times with either instrument that are solely sample dependent and have little to do with the analytical method.
For instance, metal-complexed carbons (FeC, MgC, etc.) react very slowly to acidification when determining the inorganic carbon levels and may result in analysis times over 20 minutes. Since there is no well defined “peak” during these analyses, the coulometric detector offers a distinct advantage over infrared systems because of the “absolute” nature of the analysis. The coulometric systems also offer the ability to heat and stir samples during the analysis, which aids the reaction process.
Infrared-based systems have an average analysis time of around 3 minutes for the determination of TOC by a combustion method. The coulometric-based systems offered by UIC have an average analysis time of about 7 minutes for the same analysis. There will be variations in analysis times with either instrument that are solely sample dependent and have little to do with the analytical method.
For instance, metal-complexed carbons (FeC, MgC, etc.) react very slowly to acidification when determining the inorganic carbon levels and may result in analysis times over 20 minutes. Since there is no well defined “peak” during these analyses, the coulometric detector offers a distinct advantage over infrared systems because of the “absolute” nature of the analysis. The coulometric systems also offer the ability to heat and stir samples during the analysis, which aids the reaction process.
Benefits
In general, infrared systems have the advantages of faster analysis times and lower analytical limits.
Coulometric systems have the advantages of being “calibration free”, having higher analytical limits, having higher accuracy and precision, and having the ability to handle a wider range of sample types, matrices and sizes.
In general, infrared systems have the advantages of faster analysis times and lower analytical limits.
Coulometric systems have the advantages of being “calibration free”, having higher analytical limits, having higher accuracy and precision, and having the ability to handle a wider range of sample types, matrices and sizes.